Zeroing the Pressure Transducer on Hemodynamic Monitoring Equipment Occurs When the Displays Reads
Affiliate xiv
Cardiovascular Diagnostic Procedures
Mary E. Lough and Christine Thompson
Cardiovascular Cess and Monitoring
Bedside Hemodynamic Monitoring
Hemodynamic monitoring is at a disquisitional juncture. The technology that launched invasive hemodynamic monitoring is more than than 30 years sometime, and the search to find feasible replacement monitoring technologies that are minimal or noninvasive is intense. This has created a new challenge in critical intendance. Although the use of invasive monitoring is failing, information technology is even so employed for hemodynamically unstable patients. Critical care nurses must be knowledgeable nigh traditional hemodynamic monitoring methods and be able to employ established physiologic principles in new situations. As the technology evolves, clinicians volition utilise the same physiologic principles to the new methods to ensure prophylactic and optimal outcomes for each patient. The following discussion of hemodynamic monitoring describes both established and emerging technologies.
Equipment
A traditional hemodynamic monitoring system has four component parts equally shown in Figure 14-1 and described in the following list:
Although many different types of invasive catheters can be inserted to monitor hemodynamic pressures, all such catheters are connected to like equipment (see Fig. xiv-one). However, there remains variation in the way dissimilar hospitals configure their hemodynamic systems. The basic setup consists of the post-obit:
Heparin.
The utilise of the anticoagulant heparin added to the normal saline (NS) affluent setup to maintain catheter patency remains controversial.i,2 While many units do add heparin to affluent solutions, other critical intendance units avoid heparin because of concern well-nigh development of heparin-induced antibodies that can trigger the autoimmune condition known as heparin-induced thrombocytopenia (Hit).2 This is sometimes described as a "heparin allergy" and, when present, is associated with a dramatic drop in platelet count and thrombus formation. If heparin is used in the affluent infusion, ongoing monitoring of the platelet count is recommended.3
Affluent solutions, lines, stopcocks, and disposable transducers are inverse every 96 hours per electric current Centers for Disease Control and Prevention (CDC) guidelines.4 All the same, there is variation in practice between hospitals; some change the flush solutions every 24 hours. For this reason, it is essential to be familiar with the specific written procedures that concern hemodynamic monitoring equipment in each critical care unit. Dextrose solutions are not recommended as flush solutions in monitoring catheters.four
Leveling the Transducer.
Leveling the transducer is unlike from zeroing. This process aligns the transducer with the level of the left atrium. The purpose is to line upwardly the air–fluid interface with the left atrium to correct for changes in hydrostatic pressure in blood vessels above and below the level of the middle.five
A carpenter's level or laser-light level can be used to ensure that the transducer is parallel with the phlebostatic axis reference point. When there is a modify in the patient's position, the transducer must be leveled once more to ensure accurate hemodynamic pressure measurements are obtained.5 Errors in measurement can occur if the transducer is placed below the phlebostatic axis because the fluid in the organisation weighs on the transducer, creating boosted hydrostatic pressure, and produces a falsely high reading. For every inch the transducer is below the tip of the catheter, the fluid pressure in the organization increases the measurement by 1.87 mm Hg. For example, if the transducer is positioned 6 inches below the tip of the catheter, this falsely elevates the displayed pressure level past 11 mm Hg.
If the transducer is placed to a higher place this atrial level, gravity and lack of fluid pressure level will requite an erroneously low reading. For every inch the transducer is positioned to a higher place the catheter tip, the measurement is one.87 mm Hg less than the true value. If several clinicians are taking measurements, the reference point can be marked on the side of the patient's chest to ensure accurate measurements. Box fourteen-1 summarizes other nursing activities associated with hemodynamic monitoring.
Intra-arterial Claret Force per unit area Monitoring
Indications
Intra-arterial claret pressure monitoring is indicated for any major medical or surgical condition that compromises cardiac output (CO), tissue perfusion, or fluid book status. The system is designed for continuous measurement of three blood pressure parameters: systole, diastole, and mean arterial blood pressure (MAP). The direct arterial admission is helpful in the management of patients with astute respiratory failure who crave frequent arterial blood gas measurements.
Catheters
The size of the catheter used is proportionate to the diameter of the cannulated artery. In small arteries—such as the radial and dorsalis pedis—a 20-gauge, three.8-cm to 5.ane-cm, nontapered catheter is used well-nigh often. If the larger femoral or axillary arteries are used, a 19- or 20-gauge, 16-cm catheter is used.
The catheter insertion is usually percutaneous, although the technique varies with vessel size. Catheters are nearly often inserted in the smaller arteries, using a "catheter-over-needle" unit of measurement in which the needle is used every bit a temporary guide for catheter placement. With this method, subsequently the unit of measurement has been inserted into the artery, the needle is withdrawn, leaving the supple plastic catheter in place. Insertion of a catheter into a larger artery typically uses the Seldinger technique, which involves the following steps:
Nursing Management
Intra-arterial blood pressure monitoring is designed for continuous assessment of arterial perfusion to the major organ systems of the body. MAP is the clinical parameter most oft used to assess perfusion, because MAP represents perfusion pressure level throughout the cardiac cycle. Because one third of the cardiac cycle is spent in systole and ii thirds in diastole, the MAP calculation must reflect the greater amount of time spent in diastole.8 This MAP formula can be calculated by hand or with a calculator, where diastole times ii plus systole is divided by iii as shown in the formula beneath:

A blood force per unit area of 120/60 mm Hg produces a MAP of 80 mm Hg. Still, the bedside hemodynamic monitor may show a slightly unlike digital number because bedside monitoring computers summate the surface area under the curve of the arterial line tracingviii (Table xiv-1).
Table 14-1
HEMODYNAMIC PRESSURES AND CALCULATED HEMODYNAMIC VALUES
| HEMODYNAMIC PRESSURE | DEFINITION AND Caption | NORMAL RANGE |
| Mean arterial pressure level (MAP) | Average perfusion pressure level created by arterial claret force per unit area during the cardiac cycle. The normal cardiac cycle is one third systole and two thirds diastole. These three components are divided by 3 to obtain the average perfusion force per unit area for the whole cardiac cycle. | 70-100 mm Hg |
| Key venous pressure (CVP) | Pressure created by volume in the right side of the eye. When the tricuspid valve is open, the CVP reflects filling pressures in the right ventricle. Clinically, the CVP is often used equally a guide to overall fluid residual. | 2-v mm Hg 3-eight cm water (HiiO) |
| Left atrial pressure (LAP) | Pressure level created by the volume in the left side of the centre. When the mitral valve is open, the LAP reflects filling pressures in the left ventricle. Clinically, the LAP is used afterward cardiac surgery to decide how well the left ventricle is ejecting its volume. In general, the higher the LAP, the lower the ejection fraction from the left ventricle. | 5-12 mm Hg |
| Pulmonary avenue pressure (PAP) PA systolic (PAS) PA diastolic (PAD) PAP hateful (PAP m ) | Pulsatile force per unit area in the pulmonary avenue measured past an indwelling catheter. | PAS xx-xxx mm Hg PAD 5-x mm Hg PAP m 10-15 mm Hg |
| Pulmonary avenue occlusion pressure (PAOP)* | Pressure created by the book in the left side of the center. When the mitral valve is open, the PAOP reflects filling pressures in the pulmonary vasculature, and pressures in the left side of the heart are transmitted dorsum to the catheter "wedged" into a minor pulmonary arteriole. | 5-12 mm Hg |
| Cardiac output (CO) | Amount of blood pumped out by a ventricle over 1 infinitesimal. Clinically, it can be measured using the thermodilution CO method, which calculates CO in liters per minute (L/min). | 4-6 L/min (at rest) |
| Cardiac index (CI) | CO divided by the body surface surface area (BSA), with tailoring of CO to individual body size. A BSA conversion chart is necessary to calculate CI, which is considered more authentic than CO because it is individualized to meridian and weight. CI is measured in liters per minute per foursquare meter of BSA (Fifty/min/m2). | 2.2-four.0 50/min/m2 |
| Stroke volume (SV) | Amount of blood ejected by the ventricle with each heartbeat, expressed in milliliters (mL). Hemodynamic monitoring systems summate SV by dividing cardiac output (CO in L/min) by the heart rate (60 minutes) and so multiplying the answer by 1000 to change liters to milliliters (mL). | threescore-70 mL |
| Stroke volume index (SI) | SV indexed to the BSA. | 40-l mL/g2 |
| Systemic vascular resistance (SVR) | Mean pressure difference across the systemic vascular bed divided by blood menstruation. Clinically, SVR represents the resistance confronting which the left ventricle must pump to eject its volume. This resistance is created by the systemic arteries and arterioles. As SVR increases, CO falls. SVR is measured in Wood units or dyn·sec·cm−5. If the number of Wood units is multiplied by lxxx, the value is converted to dyn·sec·cm−5. | 10-18 Wood units or 800-1400 dyn·sec·cm−v |
| Systemic vascular resistance index (SVRI) | SVR indexed to BSA. | 2000-2400 dyn·sec·cm−5 |
| Pulmonary vascular resistance (PVR) | Mean pressure difference beyond pulmonary vascular bed divided by claret flow. Clinically, PVR represents the resistance against which the correct ventricle must pump to eject its volume. This resistance is created by the pulmonary arteries and arterioles. As PVR increases, the output from the right ventricle decreases. PVR is measured in Wood units or dyn·sec·cm−v. PVR is normally one sixth of SVR. | i.ii-3.0 Wood units or 100-250 dyn·sec·cm−v |
| Pulmonary vascular resistance index (PVRI) | PVR indexed to BSA. | 225-315 dyn·sec·cm−5/m2 |
| Left cardiac work index (LCWI) | Amount of piece of work the left ventricle does each infinitesimal when ejecting blood. The hemodynamic formula represents pressure generated (MAP) multiplied past book pumped (CO). A conversion factor is used to alter mm Hg to kilogram-meter (kg-thou). LCWI is always represented every bit an indexed book (BSA nautical chart). LCWI increases or decreases because of changes in pressure level (MAP) or volume pumped (CO). | iii.iv–4.2 kg-one thousand/m2 |
| Left ventricular stroke work index (LVSWI) | Amount of work the left ventricle performs with each heartbeat. The hemodynamic formula represents force per unit area generated (MAP) multiplied past book pumped (SV). A conversion factor is used to change mL/mm Hg to gram-meter (g-m). LVSWI is always represented as an indexed book. LVSWI increases or decreases because of changes in the force per unit area (MAP) or volume pumped (SV). | 50-62 g-m/m2 |
| Correct cardiac piece of work index (RCWI) | Amount of piece of work the right ventricle performs each minute when ejecting blood. The hemodynamic formula represents pressure generated (PAP mean) multiplied by volume pumped (CO). A conversion gene is used to change mm Hg to kilogram-meter (kg-m). RCWI is always represented as an indexed value (BSA nautical chart). Similar to LCWI, the RCWI increases or decreases considering of changes in the pressure (PAP hateful) or volume pumped (CO). | 0.54-0.66 kg-grand/chiliadii |
| Right ventricular stroke piece of work alphabetize (RVSWI) | Amount of piece of work the right ventricle does each heartbeat. The hemodynamic formula represents force per unit area generated (PAP mean) multiplied by book pumped (SV). A conversion gene is used to alter mm Hg to gram-meter (g-m). RVSWI is always represented every bit an indexed value (BSA chart). Like to LVSWI, the RVSWI increases or decreases because of changes in the force per unit area (PAP mean) or volume pumped (SV). | seven.nine-9.7 g-yard/mtwo |
*Pulmonary artery occlusion force per unit area (PAOP) was formerly chosen pulmonary capillary wedge pressure level (PCW or PCWP) or pulmonary arterial wedge pressure level (PAWP).
A MAP greater than lx mm Hg is necessary to perfuse the coronary arteries. A higher MAP may be required to perfuse the brain and the kidneys. A MAP between 70 and 90 mm Hg is ideal for the cardiac patient to decrease left ventricular (LV) workload. After a carotid endarterectomy or neurosurgery, a MAP of 90 to 110 mm Hg may be more than appropriate to increase cerebral perfusion force per unit area. Systolic and diastolic pressures are monitored in conjunction with the MAP as a further guide to the accuracy of perfusion. If CO decreases, the torso compensates by constricting peripheral vessels to maintain the blood pressure. In this situation, the MAP may remain constant only the pulse pressure level (difference between systolic and diastolic pressures) narrows. The following examples explicate this point: Mr. A: BP, ninety/70 mm Hg; MAP, 76 mm Hg Mr. B: BP, 150/xl mm Hg; MAP, 76 mm Hg Both patients have a perfusion pressure of 76 mm Hg, only they are clinically very dissimilar. Mr. A is peripherally vasoconstricted, as is demonstrated past the narrow pulse pressure (90/seventy mm Hg). His pare is cool to touch, and he has weak peripheral pulses. Mr. B has a wide pulse force per unit area (150/twoscore mm Hg), warm skin, and normally palpable peripheral pulses. Nursing assessment of the patient with an arterial line includes comparison of clinical findings with arterial line readings, including perfusion pressure and MAP. If the arterial line becomes unreliable or dislodged, a cuff pressure can exist used equally a reserve system.ten In the normotensive, normovolemic patient, little deviation exists between the arm cuff blood pressure level and the intravascular catheter pressure, and differences of v to 10 mm Hg practise not by and large modify clinical management. The situation is different if the patient has a depression CO or is in stupor. The concern is that the cuff pressure may exist unreliable because of peripheral vasoconstriction, and an arterial line is generally required. It is usual practise to compare a cuff pressure after the arterial line is inserted. A recent study in hypotensive patients found that a MAP calculated from the arm cuff claret force per unit area was comparable to the intravascular arterial MAP.eleven However, blood force per unit area cuffs placed on the thigh or ankle were less accurate in hypotensive patients.11
Perfusion Pressure level.
Cuff Blood Force per unit area.
Arterial Pressure Waveform Estimation
As the aortic valve opens, blood is ejected from the left ventricle and is recorded as an increase of force per unit area in the arterial organization. The highest indicate recorded is called systole. Subsequently peak ejection (systole), the force decreases, and the pressure level drops. A notch (dicrotic notch) may be visible on the downstroke of this arterial waveform, representing closure of the aortic valve. The dicrotic notch signifies the beginning of diastole. The remainder of the downstroke represents diastolic runoff of claret menses into the arterial tree. The lowest betoken recorded is called diastole. A normal arterial pressure tracing is shown in Effigy fourteen-2. Notice that electrical stimulation (QRS) is ever first and that the arterial pressure tracing follows the initiating QRS.
Pulse Deficit.
A pulse arrears occurs when the apical HR and the peripheral pulse are not equal. In the critical intendance unit, this can be seen on the bedside monitor. Normally, at that place is one arterial upstroke for each QRS, and if there are more QRS complexes than arterial upstrokes, a pulse arrears is nowadays, as shown in Figures xiv-three and 14-vi. To identify a pulse arrears in an unmonitored patient, a stethoscope is placed over the noon of the heart. The heartbeat can exist heard, only it cannot be felt as a radial pulse. To make up one's mind whether a pulse deficit is meaning, information technology is necessary to evaluate the clinical affect on the patient and whether any modify in MAP or pulse pressure has occurred. Generally, the more than nonperfused beats, the more serious the trouble.
Damped Waveform.
If the arterial monitor shows a low blood pressure level, it is the responsibleness of the nurse to determine whether information technology is a patient problem or a problem with the equipment, as described in Table 14-ii. A low arterial blood pressure waveform is shown in Figure 14-7. In this case, the digital readout correlated well with the patient's cuff force per unit area, confirming that the patient was hypotensive. This arterial waveform is more than rounded, without a dicrotic notch, compared with the normal waveform in Figure xiv-2. A damped (flattened) arterial waveform is shown in Figure 14-8. In this case, the patient'south cuff pressure level was significantly college than the digital readout, representing a problem with equipment. A damped waveform occurs when communication from the avenue to the transducer is interrupted and produces imitation values on the monitor and oscilloscope. Damping is caused by a fibrin "sleeve" that partially occludes the tip of the catheter, by kinks in the catheter or tubing, or by air bubbles in the system. Troubleshooting techniques (come across Table 14-2) are used to notice the origin of the problem and to remove the cause of damping.
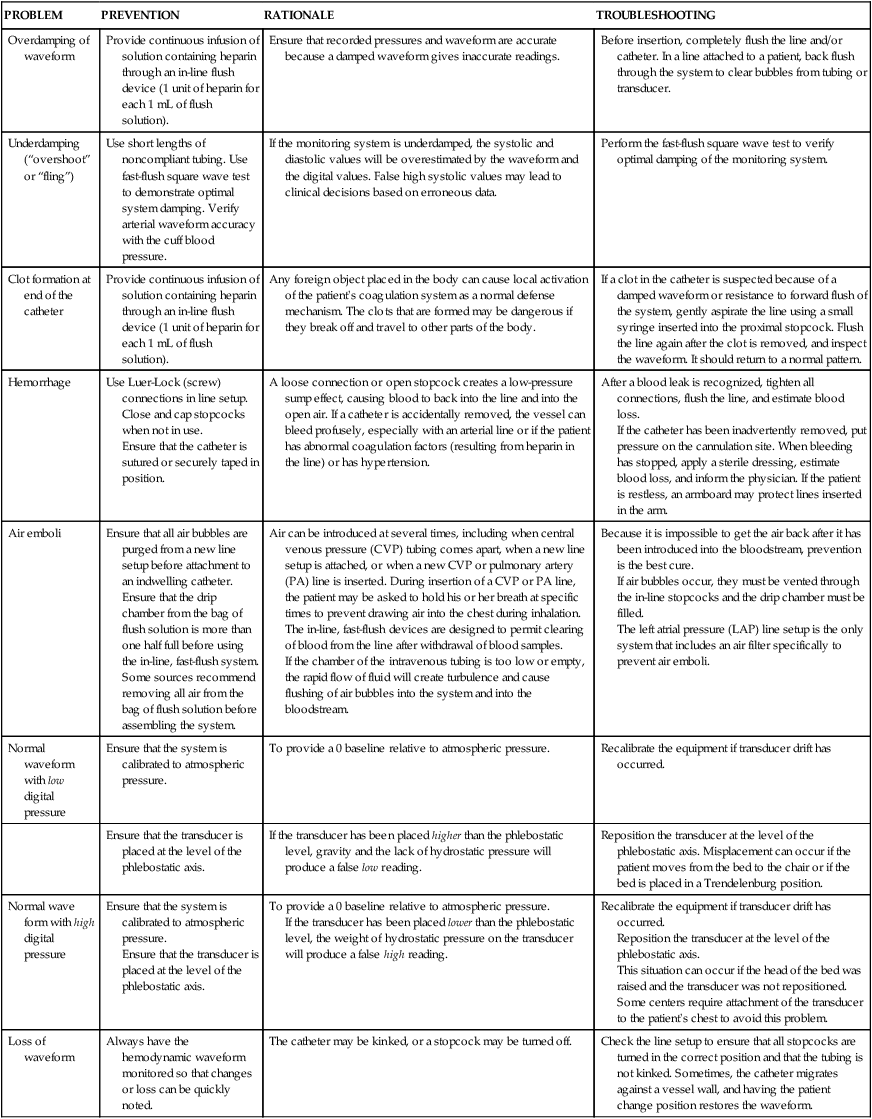
Table 14-2
NURSING MEASURES TO ENSURE PATIENT SAFETY AND TO TROUBLESHOOT Problems WITH HEMODYNAMIC MONITORING EQUIPMENT
| Problem | PREVENTION | RATIONALE | TROUBLESHOOTING |
| Overdamping of waveform | Provide continuous infusion of solution containing heparin through an in-line affluent device (1 unit of heparin for each ane mL of flush solution). | Ensure that recorded pressures and waveform are accurate considering a damped waveform gives inaccurate readings. | Earlier insertion, completely flush the line and/or catheter. In a line attached to a patient, back flush through the system to clear bubbles from tubing or transducer. |
| Underdamping ("overshoot" or "fling") | Use short lengths of noncompliant tubing. Employ fast-flush square moving ridge test to demonstrate optimal organization damping. Verify arterial waveform accurateness with the gage blood pressure. | If the monitoring system is underdamped, the systolic and diastolic values volition be overestimated past the waveform and the digital values. False high systolic values may atomic number 82 to clinical decisions based on erroneous data. | Perform the fast-flush square wave test to verify optimal damping of the monitoring system. |
| Clot formation at end of the catheter | Provide continuous infusion of solution containing heparin through an in-line flush device (one unit of measurement of heparin for each 1 mL of flush solution). | Any foreign object placed in the trunk can cause local activation of the patient's coagulation system as a normal defense mechanism. The clots that are formed may be dangerous if they break off and travel to other parts of the body. | If a clot in the catheter is suspected because of a damped waveform or resistance to forward affluent of the system, gently aspirate the line using a small syringe inserted into the proximal stopcock. Affluent the line again after the clot is removed, and audit the waveform. It should render to a normal pattern. |
| Hemorrhage | Employ Luer-Lock (screw) connections in line setup. Close and cap stopcocks when non in use. Ensure that the catheter is sutured or securely taped in position. | A loose connection or open up stopcock creates a low-pressure sump effect, causing claret to back into the line and into the open air. If a catheter is accidentally removed, the vessel can bleed profusely, especially with an arterial line or if the patient has abnormal coagulation factors (resulting from heparin in the line) or has hypertension. | After a claret leak is recognized, tighten all connections, affluent the line, and judge claret loss. If the catheter has been inadvertently removed, put pressure level on the cannulation site. When bleeding has stopped, apply a sterile dressing, estimate claret loss, and inform the doctor. If the patient is restless, an armboard may protect lines inserted in the arm. |
| Air emboli | Ensure that all air bubbling are purged from a new line setup before attachment to an indwelling catheter. Ensure that the drip chamber from the bag of flush solution is more than one half total before using the in-line, fast-affluent organization. Some sources recommend removing all air from the handbag of flush solution before assembling the organization. | Air tin can be introduced at several times, including when central venous pressure (CVP) tubing comes autonomously, when a new line setup is attached, or when a new CVP or pulmonary artery (PA) line is inserted. During insertion of a CVP or PA line, the patient may be asked to hold his or her jiff at specific times to forbid drawing air into the chest during inhalation. The in-line, fast-flush devices are designed to allow clearing of blood from the line after withdrawal of blood samples. If the chamber of the intravenous tubing is also low or empty, the rapid menses of fluid will create turbulence and crusade flushing of air bubbles into the arrangement and into the bloodstream. | Considering it is incommunicable to get the air dorsum after it has been introduced into the bloodstream, prevention is the best cure. If air bubbles occur, they must be vented through the in-line stopcocks and the baste bedroom must exist filled. The left atrial force per unit area (LAP) line setup is the only arrangement that includes an air filter specifically to prevent air emboli. |
| Normal waveform with low digital force per unit area | Ensure that the system is calibrated to atmospheric force per unit area. | To provide a 0 baseline relative to atmospheric pressure. | Recalibrate the equipment if transducer migrate has occurred. |
| Ensure that the transducer is placed at the level of the phlebostatic axis. | If the transducer has been placed college than the phlebostatic level, gravity and the lack of hydrostatic pressure level will produce a false low reading. | Reposition the transducer at the level of the phlebostatic axis. Misplacement can occur if the patient moves from the bed to the chair or if the bed is placed in a Trendelenburg position. | |
| Normal wave form with high digital pressure | Ensure that the system is calibrated to atmospheric pressure level. Ensure that the transducer is placed at the level of the phlebostatic centrality. | To provide a 0 baseline relative to atmospheric pressure. If the transducer has been placed lower than the phlebostatic level, the weight of hydrostatic pressure on the transducer will produce a false high reading. | Recalibrate the equipment if transducer drift has occurred. Reposition the transducer at the level of the phlebostatic axis. This situation can occur if the head of the bed was raised and the transducer was not repositioned. Some centers require attachment of the transducer to the patient's chest to avert this problem. |
| Loss of waveform | Always have the hemodynamic waveform monitored so that changes or loss can be chop-chop noted. | The catheter may exist kinked, or a stopcock may be turned off. | Check the line setup to ensure that all stopcocks are turned in the correct position and that the tubing is not kinked. Sometimes, the catheter migrates against a vessel wall, and having the patient change position restores the waveform. |
Fast-Flush Square Waveform Examination.
The monitoring organisation's dynamic response can exist verified for accuracy at the bedside by the fast-flush square waveform test, besides chosen the dynamic frequency response test. v The nurse performs this test to ensure that the patient pressures and waveform shown on the bedside monitor are authentic.5 The test makes use of the transmission flush organisation on the transducer. Normally, the flush device allows simply 3 mL of fluid/60 minutes. With the normal waveform displayed, the manual fast-flush procedure is used to generate a rapid increment in pressure level, which is displayed on the monitor oscilloscope. Every bit shown in Effigy fourteen-10, the normal dynamic response waveform shows a square blueprint with i or two oscillations before the return of the arterial waveform. If the system is overdamped, a sloped (rather than square) design is seen. If the system is underdamped, additional oscillations—or vibrations—are seen on the fast-flush square wave exam. This examination tin can be performed with any hemodynamic monitoring system. If air bubbles, clots, or kinks are in the system, the waveform becomes damped, or flattened, and this is reflected in the square waveform effect.
This is an piece of cake test to perform, and information technology should be incorporated into nursing care procedures at the bedside when the hemodynamic system is beginning set upward, at least in one case per shift, after opening the arrangement for any reason, and when there is concern about the accurateness of the waveform.5 If the force per unit area waveform is distorted or the digital display is inaccurate, the troubleshooting methods described in Table 14-two can be implemented. The nurse caring for the patient with an arterial line must be able to assess whether a low MAP or narrowed perfusion pressure represents decreased arterial perfusion or equipment malfunction. Assessment of the arterial waveform on the oscilloscope, in combination with clinical assessment, and use of the square waveform exam will yield the answer.
Central Venous Pressure level Monitoring
Indications
CVP monitoring is indicated whenever a patient has meaning alteration in fluid volume (run across Tabular array 14-1). The CVP tin can be used equally a guide in fluid volume replacement in hypovolemia and to assess the impact of diuresis after diuretic assistants in the case of fluid overload. When a major intravenous line is required for book replacement, a CVC is a adept selection considering large volumes of fluid can easily be delivered.
Central Venous Catheters
A range of CVC options are bachelor every bit single-, double-, triple-, and quad-lumen infusion catheters, depending on the specific needs of the patient. CVCs are fabricated from a diverseness of materials ranging from polyurethane to silicone; most are soft and flexible. Catheters that are antimicrobial-impregnated or heparin-coated accept a lower rate of bloodstream infections.4
Insertion.
The large veins of the upper thorax—subclavian (SC) and internal jugular (IJ)—are nigh commonly used for percutaneous CVC line insertion.iv The femoral vein in the groin is used when the thoracic veins are not accessible. All three major sites have advantages and disadvantages.
Femoral Vein.
The femoral vein is considered the easiest cannulation site because there are no curves in the insertion route. The big bore of the femoral vein carries a high blood flow that is advantageous for specialized procedures such equally continuous renal replacement therapy (CRRT) or plasmapheresis. Because at that place is a higher rate of nosocomial infection with femoral catheters, this site is non recommended.4 If a femoral venous access has been used, the CVC should be changed to either the SC or IJ location every bit soon every bit the patient is hemodynamically stable.iv
During insertion of a catheter in the SC or IJ vein, the patient may be placed in a Trendelenburg position. Placing the head in a dependent position causes the IJ veins in the cervix to become more than prominent, facilitating line placement. To minimize the risk of air embolus during the procedure, the patient may be asked to "take a deep breath and hold it" any time the needle or catheter is open up to air. The tip of the catheter is designed to remain in the vena cava and should not drift into the right atrium. Because many patients are awake and warning when a CVC is inserted, a brief explanation about the procedure can minimize patient feet and result in cooperation during the insertion. This cooperation is of import, considering CVC insertion is a sterile process and because the supine or Trendelenburg position may non be comfy for many patients. The electrocardiogram (ECG) should be monitored during CVC insertion because of the associated risk of dysrhythmias.
All central catheters are designed for placement past percutaneous injection after skin grooming and administration of a local anesthetic. Visualization of the vessel with a bedside ultrasound before insertion is recommended to reduce the number of CVC placement attempts.4 A prepackaged CVC kit typically is used for the procedure. The standard CVC kit contains sterile towels, chlorhexidine and alcohol for skin preparation, a needle introducer, a syringe, guidewire, and a catheter. The Seldinger technique, in which the vein is located past using a "seeking" needle and syringe, is the preferred method of placement. A guidewire is passed through the needle, the needle is removed, and the catheter is passed over the guidewire. After the tip of the catheter is correctly placed in the vena cava, the guidewire is removed. A sterile intravenous tubing and solution is attached, and the catheter is sutured in place. Following upper thoracic CVC placement, a chest radiograph is obtained to verify placement and the absence of an iatrogenic hemothorax or pneumothorax, specially if the SC vein was accessed.
Central Venous Catheter Complications
The CVC is an essential tool in care of the critically ill patient, but it is associated with some risks, and it is the responsibility of all clinicians to be informed about these hazards and to follow hospital procedures to avoid iatrogenic complications. CVC complications include air embolus, catheter-associated thrombus formation, and infection.
Air Embolus.
The run a risk of air embolus, although uncommon, is ever present for the patient with a primal venous line in identify. Air can enter during insertionxv through a disconnected or cleaved catheter past means of an open stopcock, or air tin can enter along the path of a removed CVC.16,17 This is more likely if the patient is in an upright position, because air can exist pulled into the venous system with the increase in negative intrathoracic pressure during inhalation. If a large book of air is infused rapidly, it may become trapped in the correct ventricular outflow tract, stopping blood flow from the right side of the heart to the lungs. Based on beast studies, this volume is approximately 4 mL/kg.18 If the air embolus is large, the patient will experience respiratory distress and cardiovascular collapse. An auscultatory clinical sign specifically associated with a big venous air embolism is the mill bicycle murmur.fifteen,xvi,19 A mill wheel murmur is a loud, churning audio heard over the middle chest, acquired by the obstruction to right ventricular outflow. Treatment involves immediately occluding the external site where air is entering, administering 100% oxygen, and placing the patient on the left side with the head downward (left lateral Trendelenburg position).19 This position displaces the air from the right ventricular outflow tract to the apex of the heart, where the air may exist aspirated by catheter intervention or gradually absorbed by the bloodstream every bit the patient remains in the left lateral Trendelenburg position. Precautions to preclude an air embolism in a CVP line include using only screw (Luer-Lock) connections, fugitive long loops of intravenous tubing, and using closed-top spiral caps on the three-way stopcock.
Thrombus Formation.
Clot formation (thrombus) at the CVC site is unfortunately mutual. Thrombus germination is not uniform; information technology may involve development of a fibrin sleeve around the catheter,20 or the thrombus may exist attached directly to the vessel wall. Other factors that promote jell formation include rupture of vascular endothelium, interruption of laminar blood flow, and concrete presence of the catheter, all of which activate the coagulation cascade. The take chances of thrombus formation is higher if insertion was hard or there were multiple needlesticks. Gradual thrombus formation may lead to "sudden" CVC occlusion. Ordinarily, the CVC becomes more difficult to withdraw blood from, or the CVP waveform becomes intermittently damped over a period of hours or fifty-fifty i to two days and is reported equally "needing frequent flushes" to remain patent. This state of affairs is acquired by the continued lengthening of a fibrin sleeve that extends along the catheter length from the insertion site past the catheter tip.20 Some catheters are heparin coated to reduce the hazard of thrombus formation, although the run a risk of Hitting does not brand this a benign option. Sometimes, CVC complications are additive; for example, the adventure of catheter-related infection is increased in the presence of thrombi, where the thrombus likely serves every bit a civilization medium for bacterial growth. Because of concerns over the development of Hit many hospitals apply a saline-merely flush to maintain CVC patency.21,22
Source: https://nursekey.com/cardiovascular-diagnostic-procedures/
Post a Comment for "Zeroing the Pressure Transducer on Hemodynamic Monitoring Equipment Occurs When the Displays Reads"